Perhaps one of the biggest health myths propagated in western culture and certainly in the United States, is the correlation between elevated cholesterol and cardiovascular disease (CVD). Unfortunately, despite dozens of studies, cholesterol has not been shown to actually cause CVD. To the contrary, cholesterol is vital to our survival, and trying to artificially lower it can have detrimental effects, particularly as we age.
Cholesterol seems to be one of those things that strikes fear into the hearts of many, so to speak. We have become obsessed with eating foods low in cholesterol and fat. Ask almost anyone, and they can tell you their cholesterol levels.
What is certain is that the 'little knowledge' that the media often imparts means many folks assume cholesterol is simply a 'bad' thing. Alternately, a good number of us may have heard the terms 'good' cholesterol and 'bad' cholesterol bandied about without knowing much about what this really means. In fact it is a fairly safe bet that if you asked anyone on the street for his or her instinctive response, if asked about cholesterol, they would probably say that we simply need to 'reduce it'.
The 'noddy-science' offered by marketing men to a generally scientifically-naive public has led many people to believe that we should replace certain food choices with specially developed products that can help 'reduce cholesterol'. Naturally this comes at a price and requires those who can afford it to pay maybe four or five times what a 'typical ordinary' product might cost. But is this apparent 'blanket need' to strive towards lowering our cholesterol justified? And, indeed, is it healthy?
For anyone who has had the official diagnosis of 'high cholesterol' in their bloodstream, they may even have embarked upon a program of medicinal intervention. In fact it is quite likely that they may have joined the legions of long-term pill-poppers who are already lining the pockets of the profit-oriented pharmaceutical giants.
But let's take a moment, now, to review some of the facts and fallacies about the much-maligned substance: cholesterol.
Cholesterol is needed to make hormones. Without it we would not produce estrogen, progesterone or testosterone. It is vital for the functioning of nerve synapses and provides the structural integrity for our cell membranes. Cholesterol is used by the skin to help prevent water evaporation and to make our skin waterproof. Vitamin D is synthesized from cholesterol. And bile, used for fat digestion, consists mostly of cholesterol. The liver produces about 90 percent of the cholesterol in our bodies; only 10 percent comes from diet. If we eat too much cholesterol, the liver decreases the output of cholesterol.
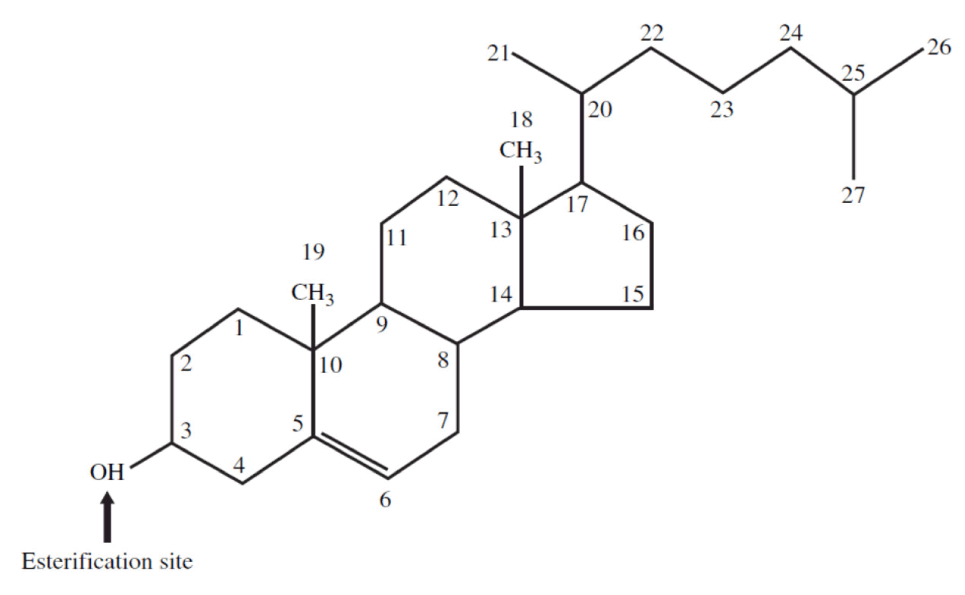
Cholesterol is a naturally occurring lipid. This means it is a type of fat or oil and it is in fact an essential component in creating and sustaining the membranes of the cells of all bodily tissues. So this alone means we need cholesterol to survive! Most of the cholesterol that is found in our bodies is actually naturally manufactured within our own cells. However there is also an additional contribution that we get from external 'nutritional' sources - the foods we consume. In a typical diet providing around 400mg of cholesterol per day from food sources, about half to two-thirds of this amount is actually absorbed through the process of digestion. The body will normally secrete about a gram (1000mg) of cholesterol per day into the bile via the ducts, and approximately three-fifths of this is then re-absorbed.
Where our tissues or organs are a particularly dense complex of cells, which have closely packed cell membranes, there will naturally be higher levels of cholesterol. The key organs that need, and contain, these higher amounts of cholesterol include the liver, the brain and the spinal cord - none of which would work well if we reduced cholesterol too much!
In effect cholesterol plays an essential role in the development and maintenance of healthy cell walls. It is also a critical factor in the synthesizing of steroid hormones, which are a key factor in our natural physical development.
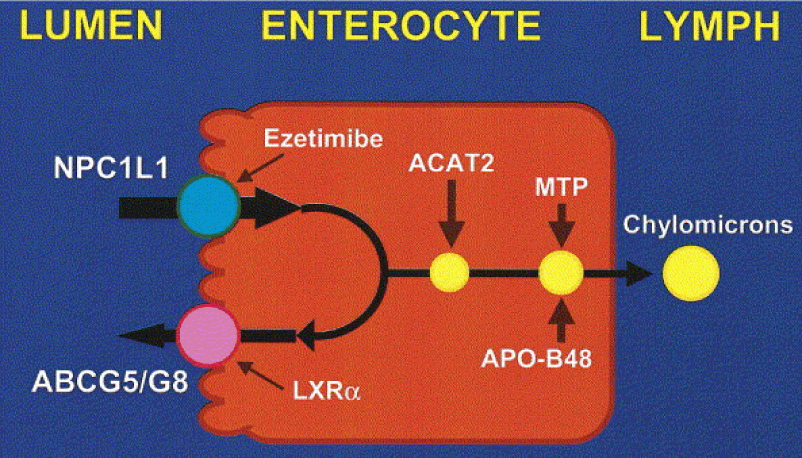
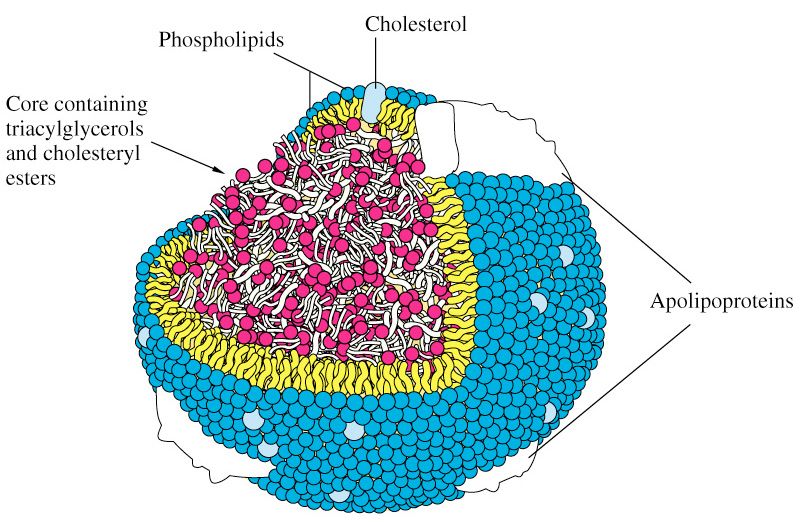
Being a lipid, cholesterol is fat-soluble, but it is not soluble in blood. However it needs to be transported around the body to the places where it can be utilized. This is why, in order to be moved around, it must become 'associated' with certain lipoproteins which feature a water-soluble (therefore 'blood transportable') coat of proteins. There are two key types of lipoproteins that transport cholesterol around the body: low-density and high-density variants. The essential cellular function of cholesterol requires that sufficient amounts are manufactured by specialized sub-systems (or organelles) within the body's cells called the endoplasmic reticulum. Alternatively, the cholesterol we need must be derived from our diet. During the process of 'digestion and assimilation' of foods, it is the low-density lipoprotein (LDL) that carries dietary cholesterol from the liver to various parts of the body.
When there is sufficient cholesterol for cellular needs, the other key transport mechanism in this amazing 'logistics system' - high-density lipoprotein (HDL) - can take cholesterol back to the liver from where any unnecessary excess can be processed for excretion.
The 'noddy-science' of the so-called 'functional food' manufacturers would have us believe that there is such a thing as 'bad' cholesterol and 'good' cholesterol. This is, in fact, totally untrue. The cholesterol itself, whether being transported by LDL or HDL, is exactly the same. Cholesterol is simply a necessary ingredient that is required to be regularly delivered around the body for the efficient healthy development, maintenance and functioning of our cells. The difference is in the 'transporters' (the lipoproteins HDL and LDL) and both types are essential for the human body's delivery logistics to work effectively.
Problems can occur, however, when the LDL particles are both small and their carrying capacity outweighs the transportation potential of available HDL. This can lead to more cholesterol being 'delivered' around the body with lower resources for returning excess capacity to the liver.
LDL can vary in its structure and occur in particles of varying size. It is the smaller LDL particle sizes that can easily become 'trapped' in the arteries by proteoglycans, which is, itself, a kind of 'filler' found between the cells in all animal and human bodies. This can then cause the cholesterol the LDL carries to contribute to the formation of fatty deposits called 'plaques' (a process known as atherogenesis). As these deposits build up, they restrict the arteries' width and flexibility. This causes an increase in blood pressure and can also lead to other cardiovascular problems such as heart attacks or strokes.
The LDL itself is consequently sometimes referred to as 'bad cholesterol', but you can now appreciate the fact that this is simply incorrect. In fact LDL, HDL and cholesterol are all essential to our health. However, it seems that it has become common for humans to have a preponderance of 'unhealthily' small LDL particles, which can become a precursor to heart and arterial disease due to the mechanisms described. It is apparently healthier to have a smaller number of larger LDL particles carrying the same quantity of cholesterol than a large number of small LDL particles might transport, but for some reason this is less common. This is an interesting area that demands more research.
When LDL becomes retained by the glycol-proteins in the arteries it is subject to being oxidized by 'free radicals'. This is when the process can become health threatening. It has therefore been suggested that increasing the amount of antioxidants in our diet might effectively 'mop up' free radicals, and consequently reduce this harmful oxidation. Although the idea of consuming foods rich in antioxidants, or even using supplements, is now widely promoted, the scientific evidence for their efficacy still remains to be fully established.
Another point to consider is the occurrence of substances called 'very-low-density-lipids' or VLDL, also known as triglycerides. VLDL is converted to LDL in the bloodstream and therefore contributes towards increased levels of LDL and to subsequent potential cholesterol-related health problems. This is why triglycerides are usually measured when a cholesterol test of your blood is undertaken.
The production of VLDL in the liver - which amounts to a combination of cholesterol and low-density apolipoprotein - is exacerbated by the intake of fructose. Fructose is the type of sugar found in many fruits, it is also a component of sucrose and of the widely used food ingredient high-fructose corn syrup. This implies that anyone whose LDL or triglyceride levels are unduly high should cut back on those sweet sugary snacks, and even on the sweeter, fructose laden fruits; not simply reduce their intake of fatty foods!
Vitamin B3, otherwise known as niacin, on the other hand, actually lowers the amount of VLDL, and therefore also LDL. In addition, niacin helps to stimulate the production of helpful HDL, the lipoprotein that carries excess cholesterol back to the liver for excretion. However, in keeping with the best traditions of consuming 'all things in moderation', currently recommended upper limits for daily intake of niacin is 35mg, given that it can have toxic effects in larger amounts. Even so, medical professionals have been known to prescribe niacin in doses as high as 2g, up to three times a day, for treatment of those with dangerously high blood cholesterol levels. Naturally you should never self-medicate with high doses of niacin without taking appropriate medical advice.
Niacin in the diet is typically derived from high protein foods including liver and other meats, as well as significant amounts being found in certain nuts and whole grains.
However one of the fashionable types of pharmaceutical drugs of recent times, introduced to treat the apparently increasing incidence of high cholesterol levels particularly in the West, are Statins. Most likely you have a friend or relative taking these useless drugs (Lipitor, Mevecor, Crestor, etc.) to lower cholesterol. Statin medications are the number-one-selling drugs in the world. They work by interfering with the liver function and reducing the production of LDL. But Statins are a questionable innovation on at least a couple of accounts. Firstly they are not without side-effects: they can, for example, lead to the breakdown of major muscular material, which can ultimately overwhelm the kidneys and even cause acute renal failure.
Statins also appear to reduce the body's natural levels of the vitamin-like, cellular protection agent known as Co-enzyme Q10. This benzoquinone plays an important role in cellular energy release, particularly in hard worked areas like the lungs, liver and heart. CoQ10 (as it is sometimes called) has also been shown to protect the brain against neurological degeneration. But perhaps most interestingly, with respect to cholesterol, CoQ10 also acts as an antioxidant, particularly active in protecting the system against LDL oxidation and the potential problems associated with this as described above. So whilst Statins might provide a reduction in LDL per se, they might also be causing more problems in the long-term. Naturally, as with many modern drugs, they generally have to be taken for the long-term by anyone who has been prescribed them.
What is particularly disturbing about Statins is, perhaps, the fact that they may be seen as a 'quick fix' for unhealthily high LDL, and consequently cholesterol levels throughout the body. They need to be taken over a long period - which makes them very profitable for drugs manufacturers. But they may also be prescribed without the over-arching message that in order to address any cholesterol problem 'naturally', the sufferer must change their lifestyle and diet. Statins can seem an easy option but may indeed merely be the beginning of a process where the 'negative health pay-off' is simply delayed rather than actively defused! That is not to say that in extreme cases of high blood cholesterol, or hypercholesterolemia, there may not be a useful role for Statin therapy when natural strategies fail or do not prove effective, or feasible.
In truth, and in summary, cholesterol is an important and essential substance that we need for health at a cellular level. It is most likely that any imbalance in our cholesterol transport system comes down to long-term poor dietary and exercise habits. Ensuring that we consume some extra anti-oxidant foods, along with including niacin rich foods, might well be of benefit. But it is perhaps most important to recognize that deliberate and continued levels of activity and the consumption of a healthful diet is a better solution than questionable quick-fix drugs, if we ever are diagnosed with levels of cholesterol and triglycerides that might give cause for concern.
Reference Sources 114, 136, 151, 158
============================================================================================
Read the full article here.

 There would be no benefit to society if we drastically reduce the number of heart attacks only to find out that everybody is developing cancer.
There would be no benefit to society if we drastically reduce the number of heart attacks only to find out that everybody is developing cancer.



