
This is a guest post by Peter Attia and is a summary based on a 10-part series of the same name that you can find at The Eating Academy.
To put this summary post and, more importantly, this 10-part series in perspective, let’s examine one of the most pervasive pieces of dietary advice given to people worldwide:
“Eating foods that contain any cholesterol above 0 mg is unhealthy.”
- T. Colin Campbell, PhD, author of The China Study.
No summary of this length can begin to fully address a topic as comprehensive as cholesterol metabolism and the pathogenesis of atherosclerosis. In fact, those of us who challenge conventional wisdom often find ourselves needing to do exactly what Frederic Bastiat suggested:
“We must admit that our opponents in this argument have a marked advantage over us. They need only a few words to set forth a half-truth; whereas, in order to show that it is a half-truth, we have to resort to long and arid dissertations.”
So, at the risk of trying to minimize the “long and arid” part of this process, below are the 10 things you need to know to be the judge – for yourself – if the conventional advice about cholesterol is correct.
1. The sine qua non of atherosclerosis is the presence of a sterol in an artery wall. How it gets there is the only thing we should be worrying about.
Contrary to popular belief, atherosclerosis is not caused by many of things we think of, such as smoking, high blood pressure, diabetes, high LDL (the so-called “bad” cholesterol), or low HDL (the so-called “good” cholesterol). Some of these are certainly markers of risk – low HDL, for example – while others accelerate the process – smoking, for example – but none of these are the direct cause of atherosclerosis.The sine qua non of atherosclerosis is the presence of sterols (cholesterol or phytosterol) in arterial wall macrophages. Sterols are delivered to the arterial wall by the penetration of the endothelium by an apoB-containing lipoprotein, which transport the sterols. In other words, unless an apoB-containing lipoprotein particle violates the border created by an endothelium cell and the layer it protects, the media layer, there is no way atherogenesis occurs. If this is a bit confusing, don’t worry. It’s all made clear below.
2. Cholesterol is vital for life; no cholesterol = no life.
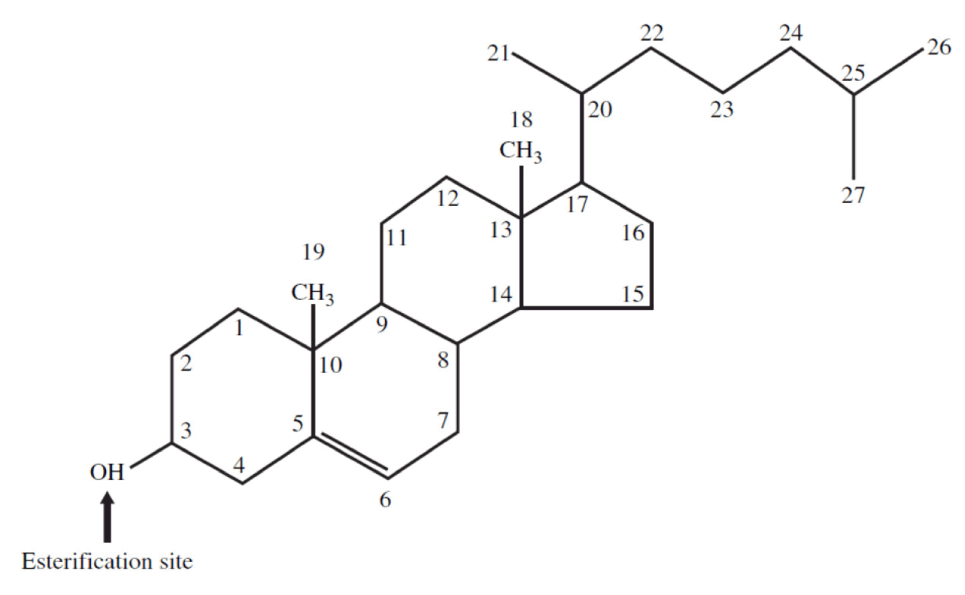
Cholesterol is a 27-carbon molecule shown in the figure below. Each line in this figure represents a bond between two carbon atoms. That’s it. Mystery over.All this talk about “cholesterol” and most people don’t actually know what it is. So, there you have it. Cholesterol is “just” another organic molecule in our body.
I need to make one distinction that will be very important later. Cholesterol, a steroid alcohol, can be “free” or “unesterified” (“UC” as we say, which stands for unesterified cholesterol) which is its active form, or it can exist in its “esterified” or storage form which we call a cholesterol ester (“CE”). The diagram below shows a free (i.e., UC) molecule of cholesterol. An esterified variant (i.e., CE) would have an “attachment” where the arrow is pointing to the hydroxyl group on carbon #3, aptly named the “esterification site.”
One of the biggest misconceptions is that cholesterol is “bad.” This could not be further from the truth. Cholesterol is very good! In fact, there are (fortunately rare) genetic disorders in which people cannot properly synthesize cholesterol. One such disease is Smith-Lemli-Opitz syndrome (also called “SLOS,” or 7-dehydrocholesterol reductase deficiency) which is a metabolic and congenital disorder leading to a number of problems including autism, mental retardation, lack of muscle, and many others.
Cholesterol is absolutely vital for our existence. Every cell in our body is surrounded by a membrane. These membranes are largely responsible for fluidity and permeability, which essentially control how a cell moves, how it interacts with other cells, and how it transports “important” things in and out. Cholesterol is one of the main building blocks used to make cell membranes (in particular, the ever-important “lipid bilayer” of the cell membrane).
Beyond cholesterol’s role in allowing cells to even exist, it also serves an important role in the synthesis of vitamins and steroid hormones, including sex hormones and bile acids. Make sure you take a look at the picture of steroid hormones synthesis and compare it to that of cholesterol (above). If this comparison doesn’t convince you of the vital importance of cholesterol, nothing I say will.
One of the unfortunate results of the eternal need to simplify everything is that we (i.e., the medical establishment) have done the public a disservice by failing to communicate that there is no such thing as “bad” cholesterol or “good” cholesterol. All cholesterol is imperative for life to exist!
The only “bad” outcome is when cholesterol ends up inside of the wall of an artery, most famously the inside of a coronary artery or a carotid artery, AND leads to an inflammatory cascade which results in the obstruction of that artery (make sure you check out the pictures in the links above). When one measures cholesterol in the blood we really do not know the final destination of those cholesterol molecules!
3. The cholesterol we eat has little to do with the cholesterol we measure in our bloodstream.
We ingest (i.e., take in) cholesterol in many of the foods we eat and our body produces (“synthesizes”) cholesterol de novo from various precursors. About 25% of our daily “intake” of cholesterol – roughly 300 to 500 mg – comes from what we eat (called exogenous cholesterol), and the remaining 75% of our “intake” of cholesterol – roughly 800 to 1,200 mg – is made by our body (called endogenous production). To put these amounts in context, consider that total body stores of cholesterol are about 30 to 40 gm (i.e., 30,000 to 40,000 mg) and most of this resides within our cell membranes. Nearly every cell in the body can produce cholesterol, and thus very few cells actually require a delivery of cholesterol. Cholesterol is required by all cell membranes and to produce steroid hormones and bile acids.Of this “made” or “synthesized” cholesterol, our liver synthesizes about 20% of it and the remaining 80% is synthesized by other cells in our bodies. The synthesis of cholesterol is a complex four-step process (with 37 individual steps) that I will not cover here, but I want to point out how tightly regulated this process is, with multiple feedback loops. In other words, the body works very hard (and very “smart”) to ensure cellular cholesterol levels are within a pretty narrow band (the overall process is called cholesterol homeostasis). Excess cellular cholesterol will crystalize and cause cellular apoptosis (programmed cell death). Plasma cholesterol levels (which is what clinicians measure with standard cholesterol tests) often have little to do with cellular cholesterol, especially artery cholesterol, which is what we really care about. For example, when cholesterol intake is decreased, the body will synthesize more cholesterol and/or absorb (i.e., recycle) more cholesterol from our gut. The way our body absorbs and regulates cholesterol is really amazing, so I want to spend a bit of time discussing it.
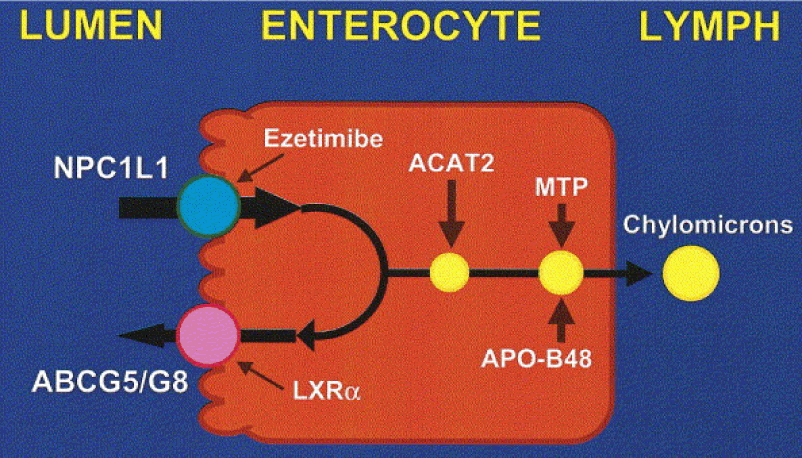
- The blue circle in this figure represents something called a Niemann-Pick C1-like 1 protein (NPC1L1). It sits at the apical surface of enterocytes and it promotes active influx (i.e., bringing in) of gut luminal unesterified cholesterol (UC) as well as unesterified phytosterols into the enterocyte. Think of this NPC1L1 as the ticket-taker at the door of the bar (where the enterocyte is the “bar”); he lets most cholesterol (“people”) in. However, NPC1L1 cannot distinguish between cholesterol (“good people”) and phytosterol (“bad people” – for reasons I won’t discuss here) or even too much cholesterol (“too many people”).
- The pink circle in this figure represents a structure called the adenosine triphosphate (ATP)-binding cassette (ABC) transporters ABCG5 and ABCG8. This structure promotes active efflux (i.e., kicking out) of unesterified sterols (cholesterol and plant sterols – of which over 40 exist) from enterocytes back into the intestinal lumen for excretion. Think of ABCG5/G8 as the bouncer at the bar; he gets rid of the really bad people (e.g., phytosterols, as they serve no purpose in humans) you don’t want in the bar who snuck past the ticket-taker (NPC1L1). Of course, in cases of hyperabsorption (i.e., where the gut absorbs too much of a good thing) they can also efflux out un-needed cholesterol. Along this analogy, once too many “good people” get in the bar, fire laws are violated and some have to go. The enterocyte has “sterol-excess sensors” (a nuclear transcription factor called LXR) that do the monitoring, and these sensors activate the genes that regulate NPC1L1 and ABCG5/G8.
- Only free or unesterified cholesterol (UC) can be absorbed through gut enterocytes. In other words, cholesterol esters (CE) cannot be absorbed because of the bulky side chains they carry.
- Much (> 50%) of the cholesterol we ingest from food is esterified (CE), hence we don’t actually absorb much, if any, exogenous cholesterol (i.e., cholesterol in food).
- Furthermore, most of the unesterified cholesterol (UC) in our gut (on the order of about 85%) is actually of endogenous origin (meaning it was synthesized in bodily cells and returned to the liver), which ends up in the gut via biliary secretion and ultimately gets re-absorbed by the gut enterocyte. The liver is only able to efflux (send out via bile into the gut) UC, but not CE, from hepatocytes (liver cells) to the biliary system. Liver CE cannot be excreted into bile. So, if the liver is going to excrete CE into bile and ultimately the gut, it needs to de-esterify it using enzymes called cholesterol esterolases which can convert liver CE to UC.
4. The cholesterol in our bloodstream has little to do with the cholesterol in our artery walls (i.e., atherosclerosis).
To understand how cholesterol travels around our body requires some understanding of the distinction between hydrophobic and hydrophilic. A molecule is said to be hydrophobic (also called nonpolar) if it repels water, while a molecule is said to be hydrophilic (also called polar) if it attracts water. Think of your veins, arteries, and capillaries as the “waterways” or rivers of your body. Cholesterol is precious “cargo” that needs to move around, but it needs a “boat” to carry it.The proteins that traffic collections of lipids are called apoproteins. Once bound to lipids they are called apolipoproteins, and the protein wrapped “vehicle” that transports the lipids are called lipoproteins. Many of you have probably heard this term before, but I’d like to ensure everyone really understands their important features. A crucial concept is that, for the most part, lipids go nowhere in the human body unless they are a passenger inside a protein wrapped vehicle called a lipoprotein. As their name suggests, lipoproteins are part lipid and part protein. They are mostly spherical structures which are held together by a phospholipid membrane (which, of course, contains free cholesterol). The figure below shows a schematic of a lipoprotein.
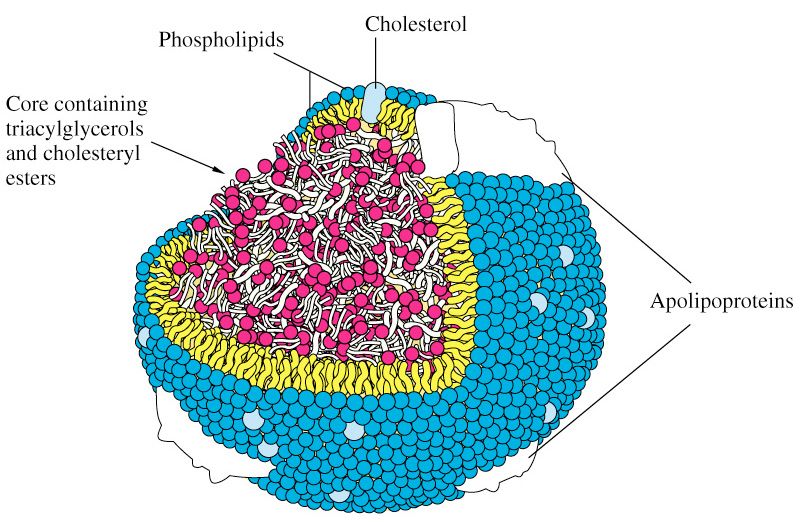
You will also notice variable-sized proteins on the surface of the lipid membrane that holds the structure together. The most important of these proteins are called apolipoproteins, as I alluded to above. The apolipoproteins on the surface of lipoprotein molecules serve several purposes including:
- Assisting in the structural integrity and solubility of the lipoprotein;
- Serving as co-factors in enzymatic reactions;
- Acting as ligands (i.e., structures that help with binding) for situations when the lipoprotein needs to interact with a receptor on a cell.
5. The only way sterols end up in artery walls – the one place we don’t want them to be – is if the sterols are carried there by an apoB-containing lipoprotein particle.
So what drives a LDL particle to do something as sinister as to leave the waterway (i.e., the bloodstream) and “illegally” try to park at a dock (i.e., behind an endothelial cell)? Well, it is a gradient driven process which is why particle number is the key driving parameter.As it turns out, this is probably a slightly less important question than the next one: what causes the LDL particle to stay there? In the parlance of our metaphor, not only do we want to know why the boat leaves the waterway to illegally park in the dock with its precious cargo, but why does it stay parked there? This phenomenon is called “retention” in lipidology-speak.
Finally, if there was some way a LDL particle could violate the endothelium, AND be retained in the space behind the cell (away from the lumen on the side aptly called the sub-endothelial space) BUT not elicit an inflammatory (i.e., immune) response, would it matter?
I don’t know. But it seems that not long after a LDL particle gets into the sub-endothelial space and takes up “illegal” residence (i.e., binds to arterial wall proteoglycans), it is subject to oxidative forces, and as one would expect an inflammatory response is initiated. The result is full blown mayhem. Immunologic gang warfare breaks out and cells called monocytes and macrophages and mast cells show up to investigate. When they arrive and find the LDL particle, they do all they can to remove it. In some cases, when there are few LDL particles, the normal immune response is successful. But, it’s a numbers game. When LDL particle invasion becomes incessant, even if the immune cells can remove some of them, it becomes a losing proposition and the actual immune response to the initial problem becomes chronic and maladaptive and expands into the space between the endothelium and the media.
The multiple-sterol-laden macrophages or foam cells coalesce, recruit smooth muscle cells, induce microvascularization, and before you know it complex, inflamed plaque occurs. Microhemorrhages and microthrombus formations occur within the plaque. Ultimately the growing plaque invades the arterial lumen or ruptures into the lumen inducing luminal thrombosis. Direct luminal encroachment by plaque expansion or thrombus formation causes the lumen of the artery to narrow, which may or may not cause ischemia.
Read more: http://www.marksdailyapple.com/the-straight-dope-on-cholesterol-10-things-you-need-to-know-part-1/#ixzz24wyQCVFe
=====================================================================
Read the complete article here.
No comments:
Post a Comment
I appreciate appropriate comments but reserve the right to publish those with credible, verifiable, significant information to contribute to the topic at hand. I will not post comments with commercial content nor those containing personal attacks. Thank You.